What Is The Purpose Of A Buffer System
What is the purpose of a buffer system. By definition a buffer is a system or area that is used to resist change and moderate fluctuations. Buffer systems are made of either a weak acid and its salt or a weak base and its salt. Buffer P1 is a resuspension buffer used when purifying plasmid DNA.
Below the buffer system represented by a chemical reaction of Hydrofluoric acid reacting with water making hydro knee mine and the consequent based fluoride. A buffer solution can protect the integrity of the proteins while separating them from other integrated cell components. The buffer is a weak acid and its conjugate base allowing the pH of the buffered solution to remain constant.
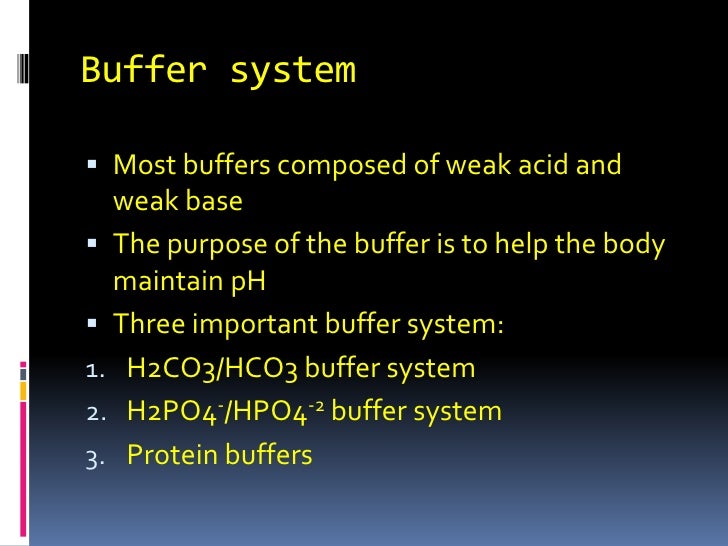
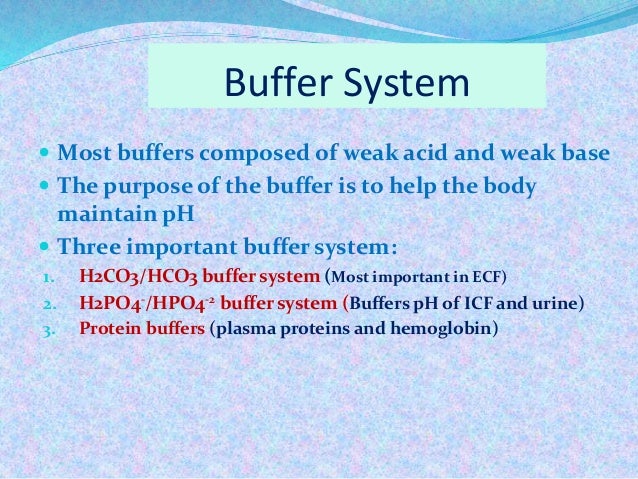
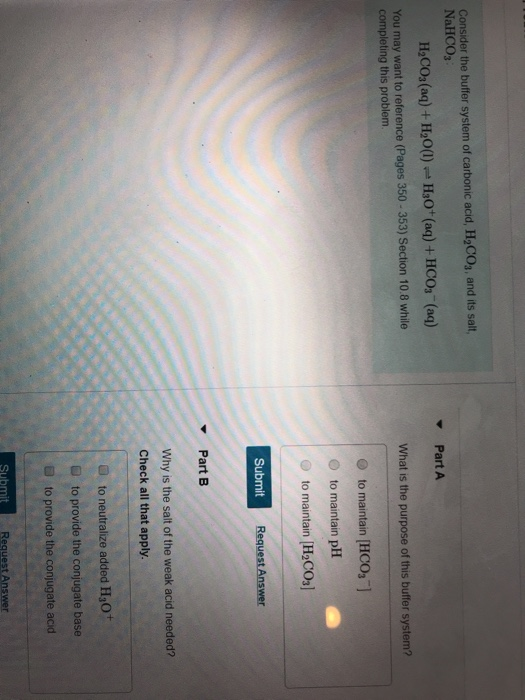
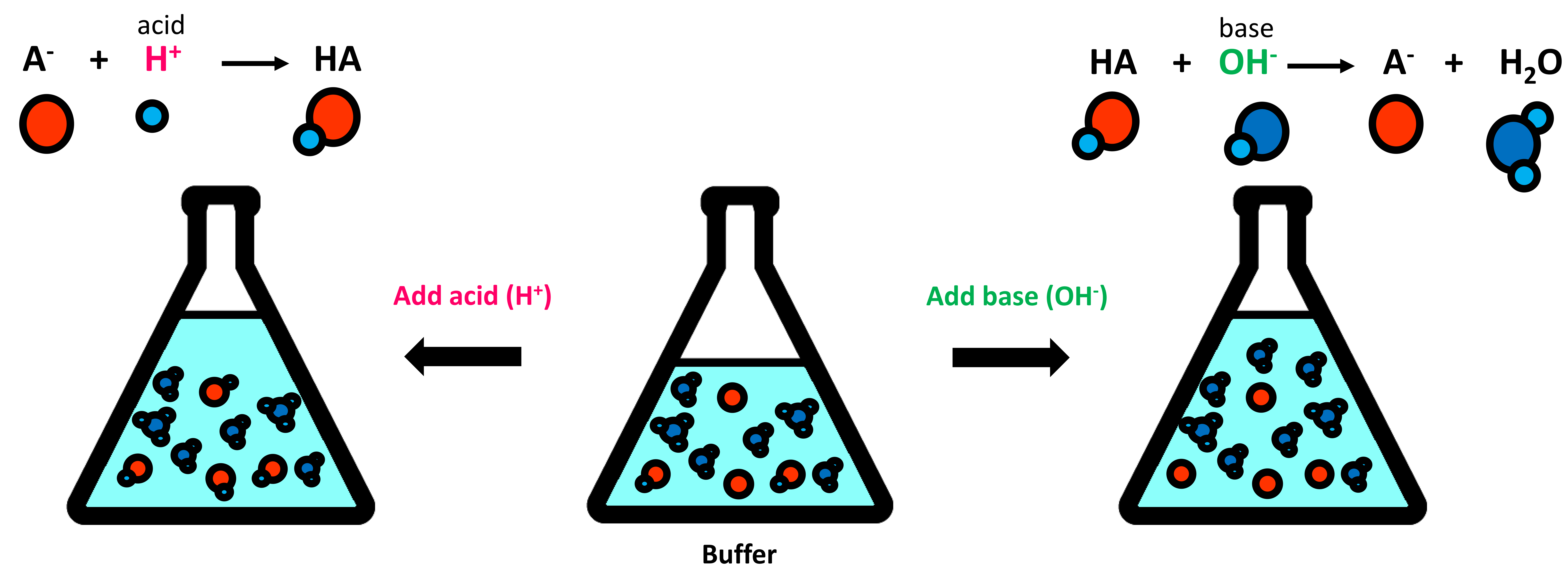
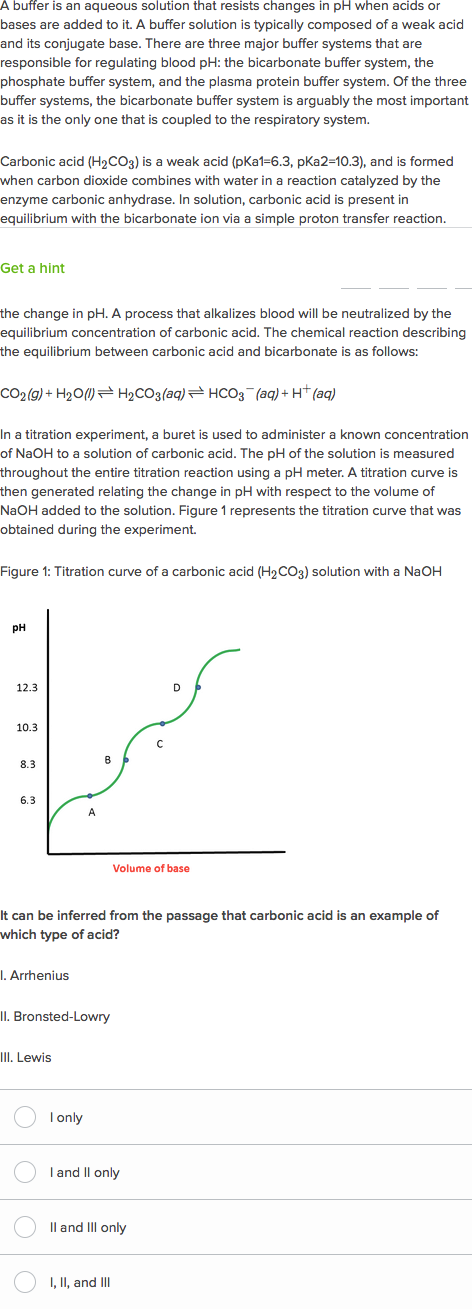
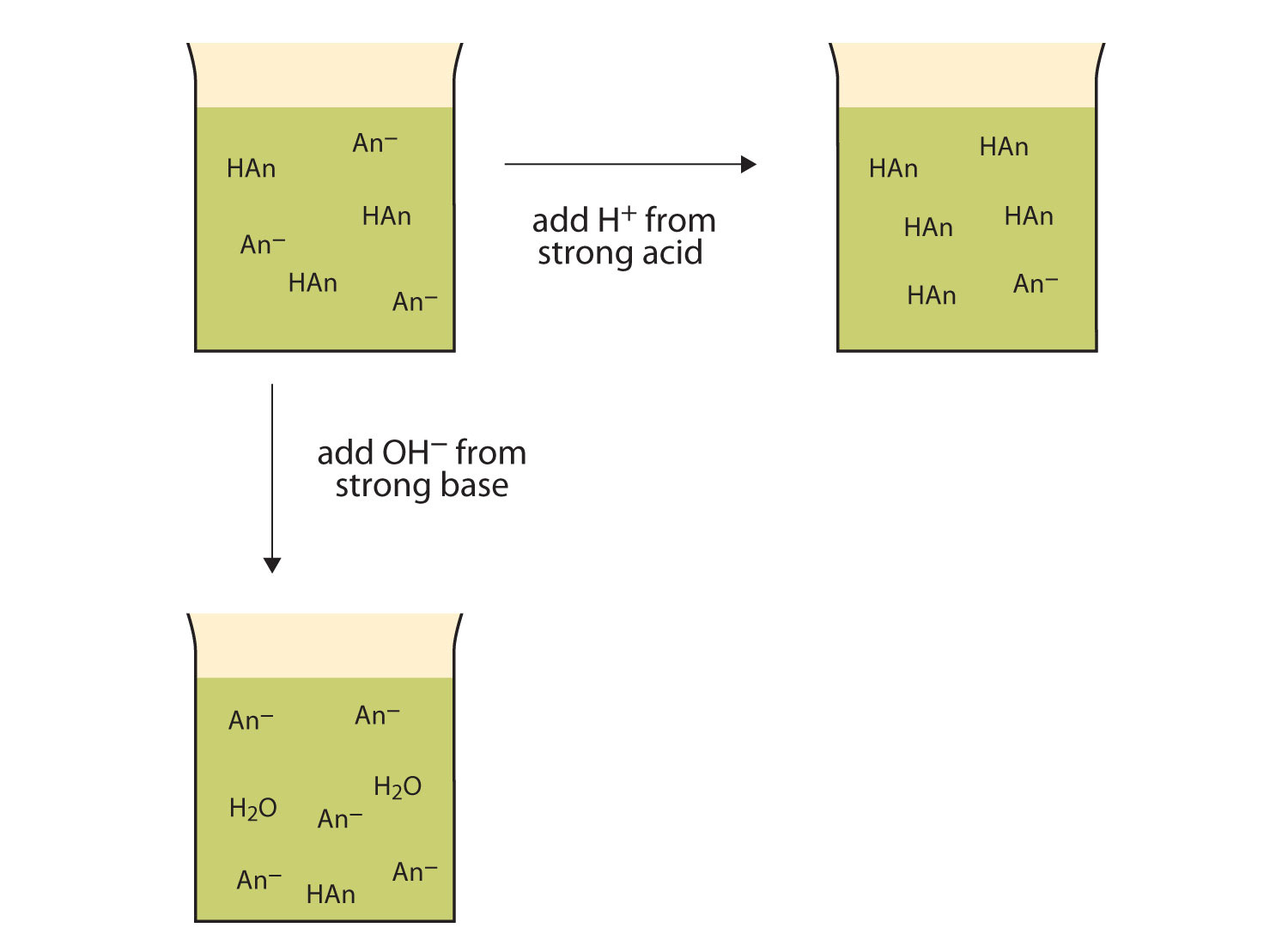
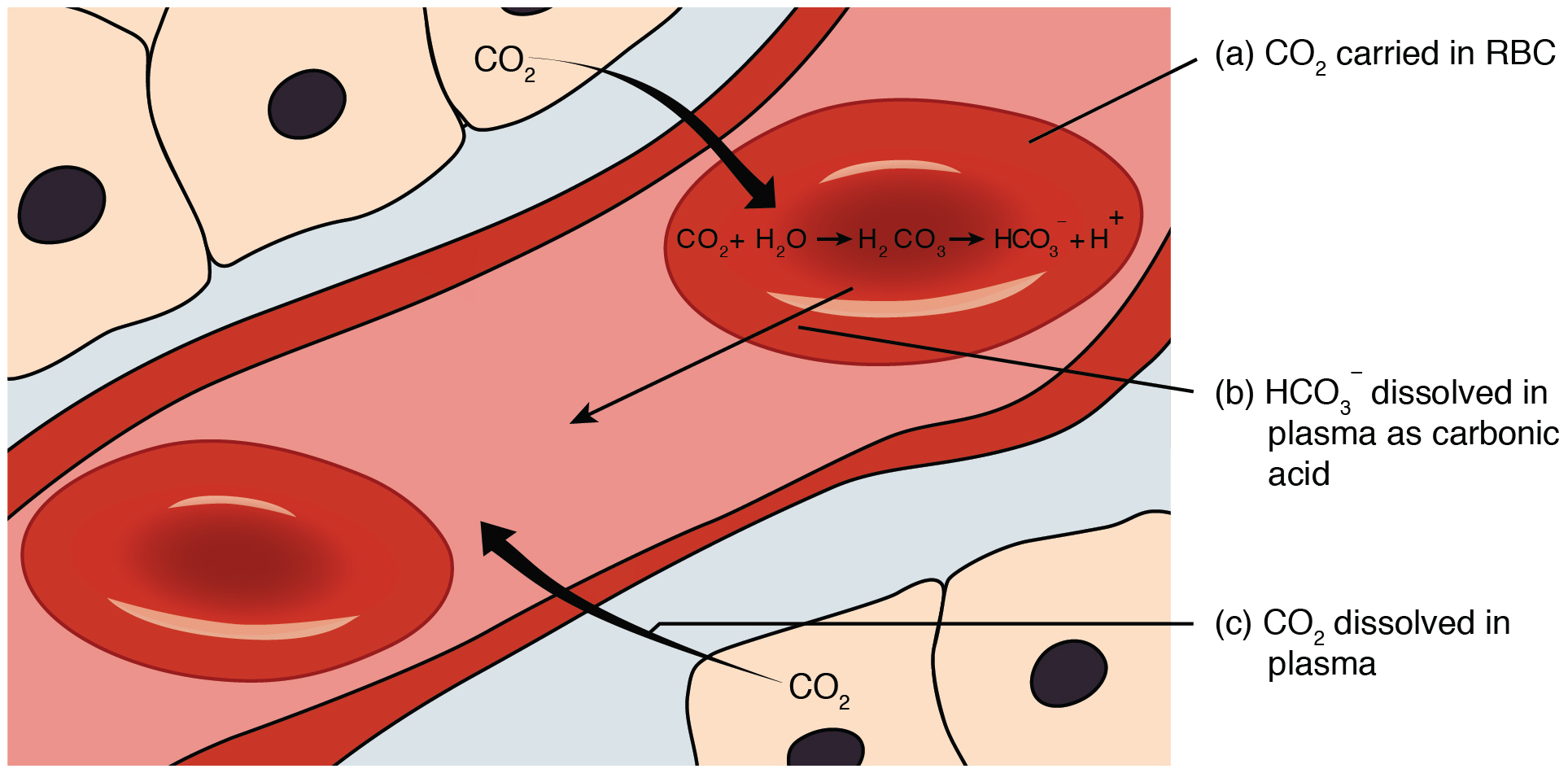
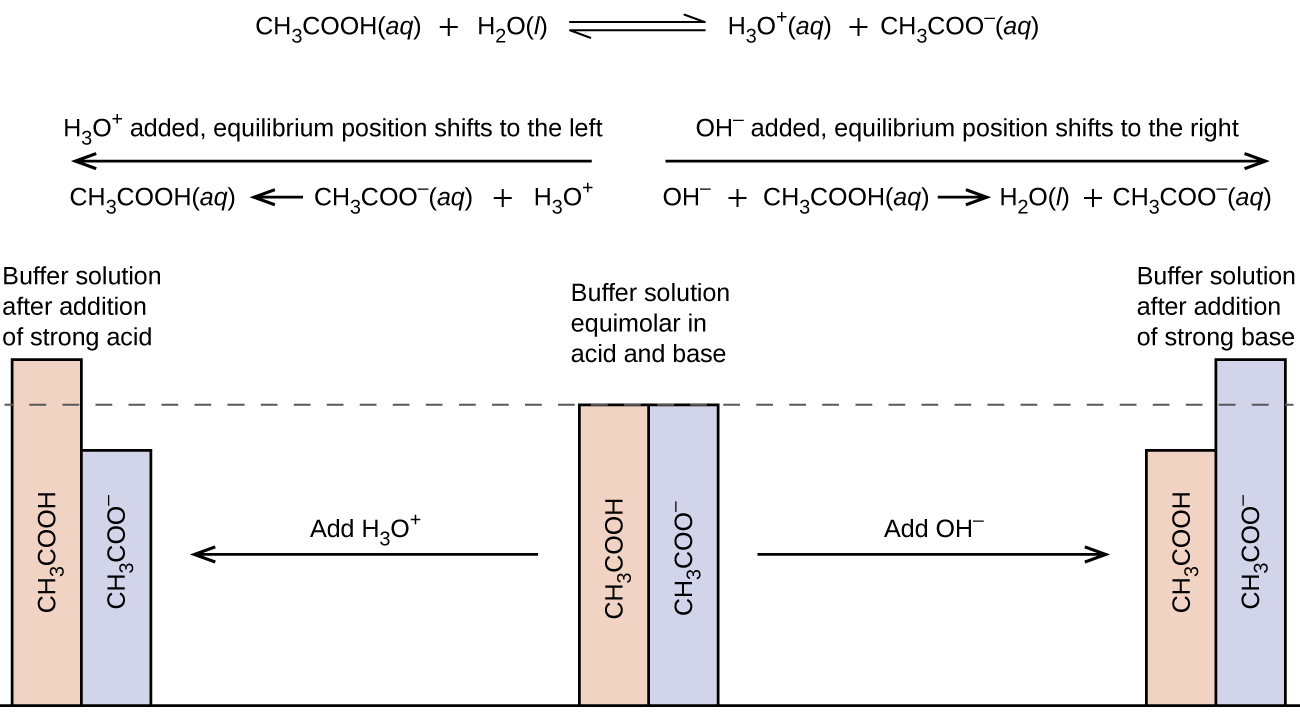
A buffer is a mixture of an acid that does not ionize completely in water and its corresponding base-for example carbonic acid H 2 CO 3 and sodium bicarbonate NaHCO 3. This happens when the concentration of the weak acid is equal to that of the conjugate base. A buffer system has the property of resisting pH changes despite additions of acid or base.
Absorb the movement of steering wheel if it is mishandled when the hydraulic pump stop in. Absorb the difference between the steering order speeds and follow up speed. When is the pH equal to the pKa of the weak acid.
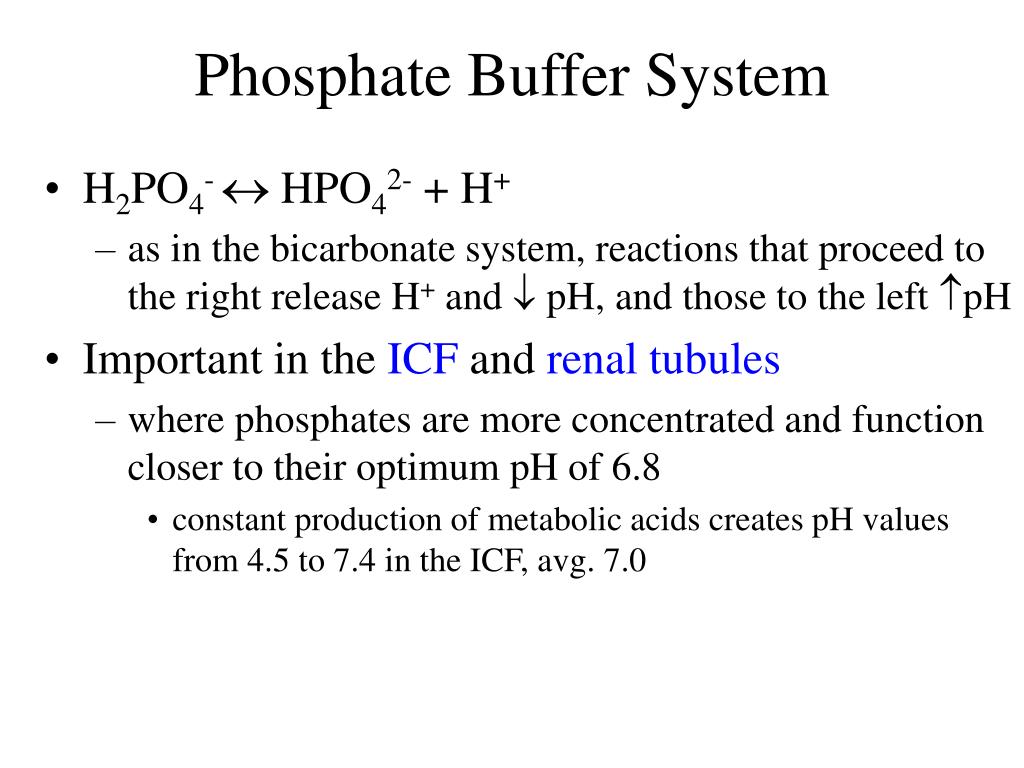
The buffer system uses its electrochemical characteristics to carry the current and protect the samples. A conjugate acid-base pair is typically composed of a weak acid and the basic ion formed when that acid loses a hydrogen ion. Phosphate buffers are widely used because they help maintain a constant pH level in a particular environment.
50 mM TrisCl pH 80. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. Considerations To accomplish this goal researchers need to choose a buffer solution thats compatible with the protein in question and recreates an ionic environment similar to the ionic environment of the cell.
Moreover what is the concentration of buffer in p1. Check all that apply.
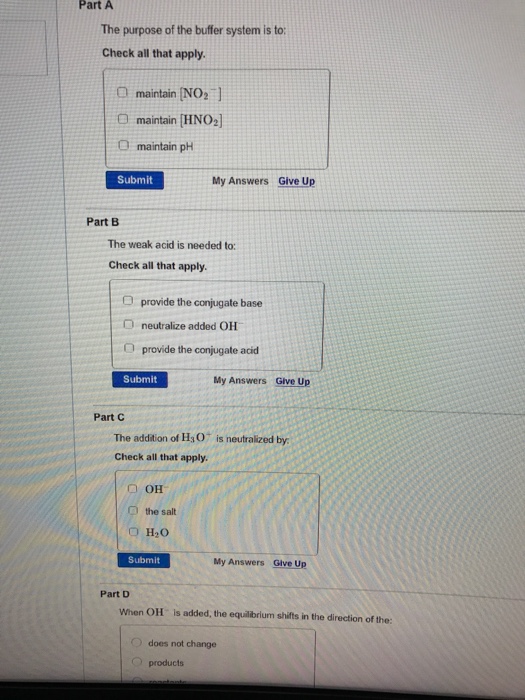
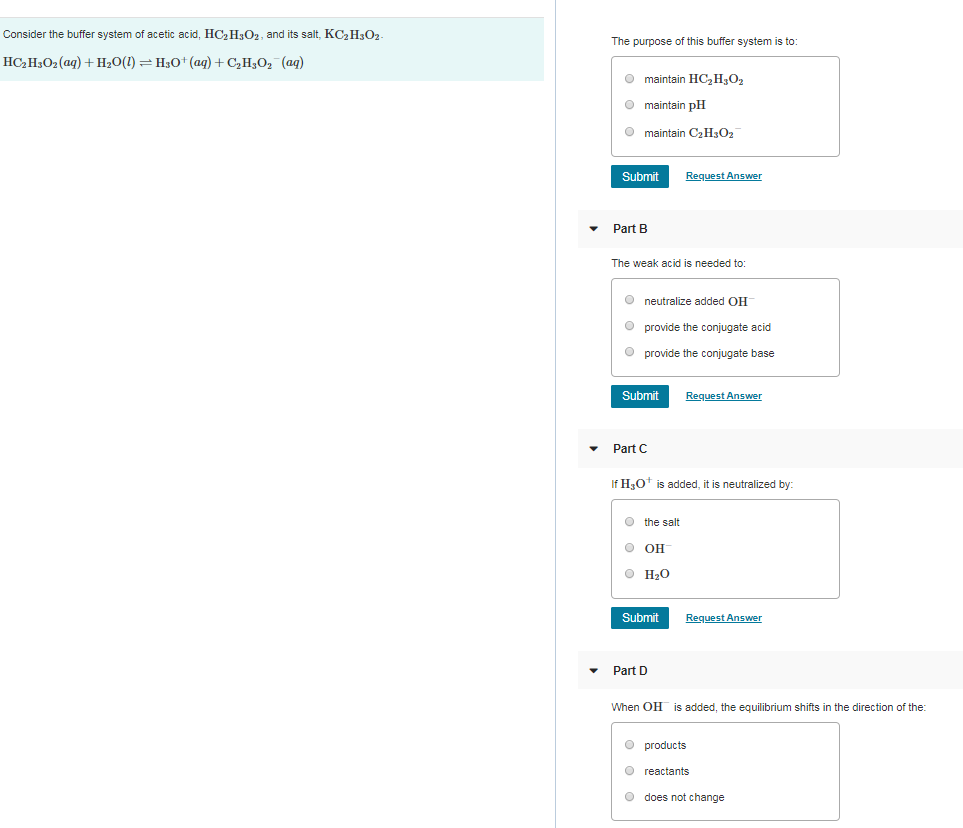
The purpose of the buffer system is to.
Buffer systems are made of either a weak acid and its salt or a weak base and its salt. But the benefits of buffered aspirin are disputed. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. In our case a buffer tank is a unit of varying size filled with water heated by a renewable energy source and adapted to increase the efficiency of that renewable energy source. Phosphate buffers are widely used because they help maintain a constant pH level in a particular environment. Without these buffer systems cellular pH and the pH of fluids outside the cells would fall. The purpose of the buffer system is to. When is the pH equal to the pKa of the weak acid. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes.
Keeping the acetylsalicylic acid in ionic form or preventing it from dissolving until it reached the small intestine would prevent it from causing bleeding. The composition of Buffer P1 is. By definition a buffer is a system or area that is used to resist change and moderate fluctuations. Phosphate buffers are widely used because they help maintain a constant pH level in a particular environment. Provide the conjugate base neutralize added OH- provide the conjugate acid. A buffer system in the human body is an interaction between a weak acid-base conjugate pair that keeps the body at the proper pH. IN FOOD INDUSTRY Buffers are also used in foods to maintain the acidity of the food in order to preserve the flavor and appearance of food.































Posting Komentar untuk "What Is The Purpose Of A Buffer System"