Which Statement Best Describes The Relationship Between Activation Energy And Rate Of Reaction
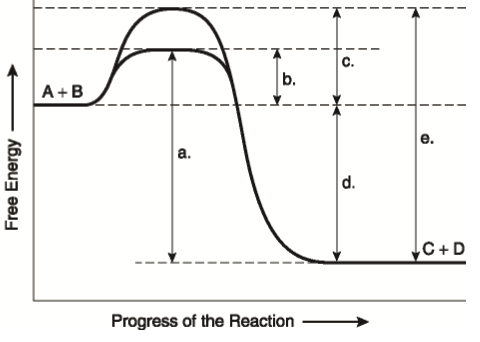
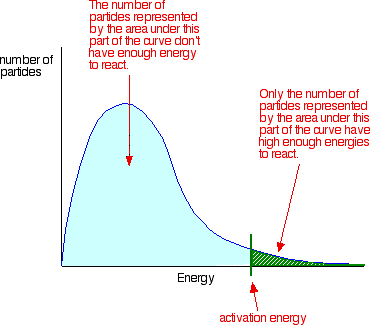
Which statement best describes the relationship between activation energy and rate of reaction. The activation energy of an exothermic reaction is negative. Increasing the activation energy can increase the rate of a reaction. In the hypothetical reaction below substance A is consumed at a rate of 20 molLs.
Cthere is no relationship between activation energy and rate of a reaction. Increasing the reaction energy does not alter the rate of reaction. Increasing the activation energy can increase the rate of a reaction.
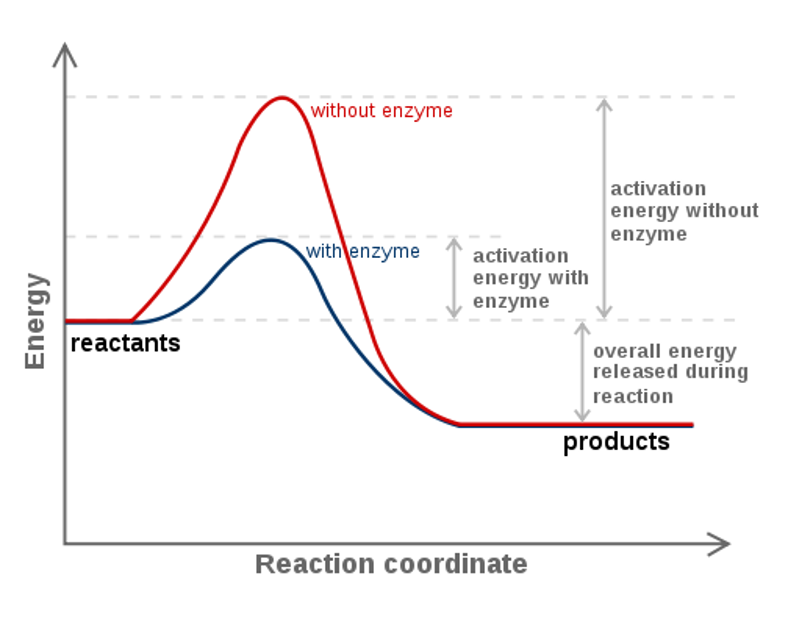
Bdecreasing the activation energy can increase the rate of a reaction. If this reaction is at dynamic equilibrium at what rate will substance B be consumed. Which type of molecule that can be found in living things lacks carbon atoms.
Which statement best describes the relationship between activation energy and rate of reaction. There is no relationship between activation energy and rate of a reaction. There is no relationship between activation energy and reaction rate.
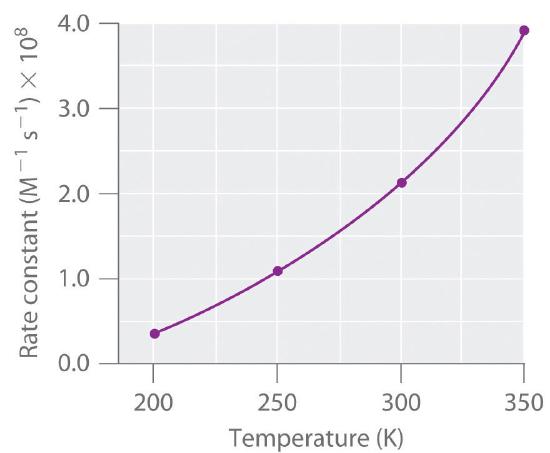
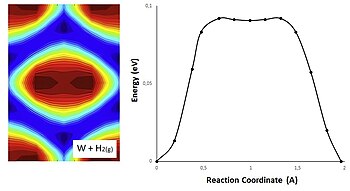
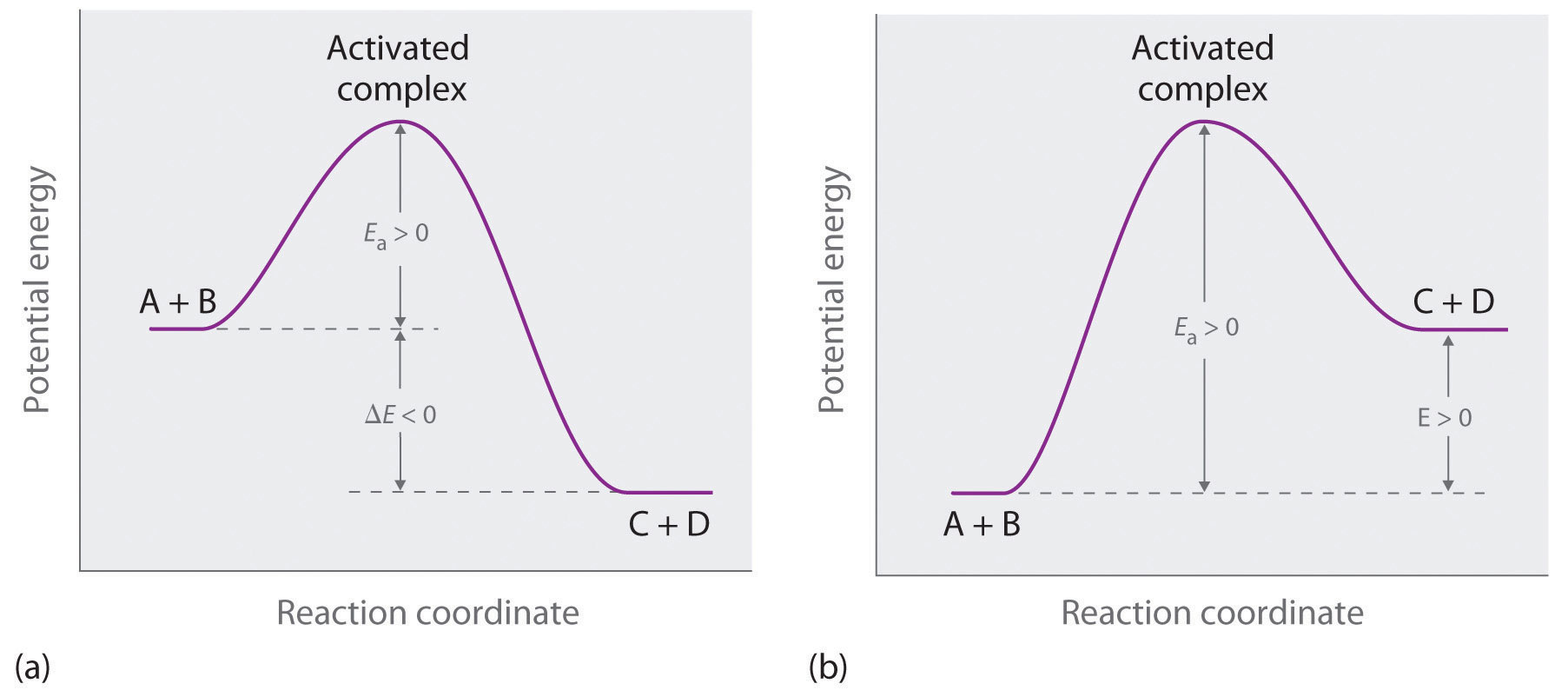
Increasing the activation energy can increase the rate of a reaction. Increasing the activation energy can increase the rate of a reaction. The Arrhenius equation describes the relationship between the rate constant k and the energy of activation Ea.
Increasing the reaction energy does not alter the rate of reaction. AIncreasing the activation energy can increase the rate of a reaction. Ddecreasing the activation energy can.
Reducing the activation energy can decrease the rate of a reaction. K AeEa RT In this equation A is an empirical constant R is the ideal-gas constant e is the base of natural logarithms and T is the absolute temperature.
Which statement is usually true about the relationship between activation energy and reaction rates.
Which statement best describes the relationship between activation energy and rate of reaction. Which statement best describes the relationship between activation energy and rate of reaction a increasing the activation energy can increase the rate of a reaction. There is no relationship between activation energy and rate of a reaction. Which statement best describes the relationship between activation energy and rate of reaction. BReducing the activation energy can increase the rate of a reaction. Increasing the activation energy can increase the rate of a reaction. There is no relationship between activation energy and rate of a reaction. There is no relationship between activation energy and rate of a reaction. K AeEa RT In this equation A is an empirical constant R is the ideal-gas constant e is the base of natural logarithms and T is the absolute temperature.
Which statement best describes the relationship between activation energy and rate of reaction. There is no relationship between activation energy and rate of a reaction. Increasing the activation energy can increase the rate of a reaction Reducing the activation energy can increase the rate of a reaction. Increasing the activation energy can increase the rate of a reaction. Increasing the activation energy can increase the rate of a reaction. Increasing the activation energy can increase the rate of a reaction. If this reaction is at dynamic equilibrium at what rate will substance B be consumed.

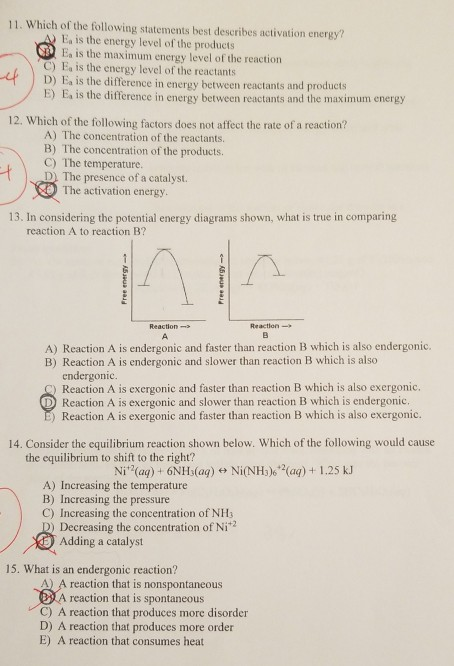


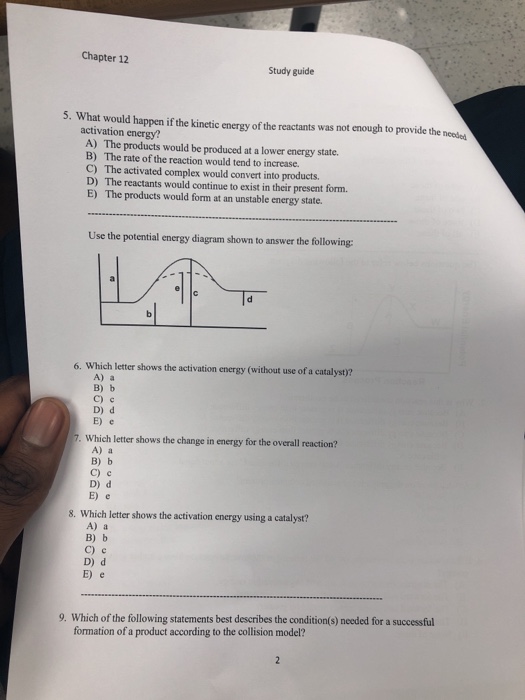
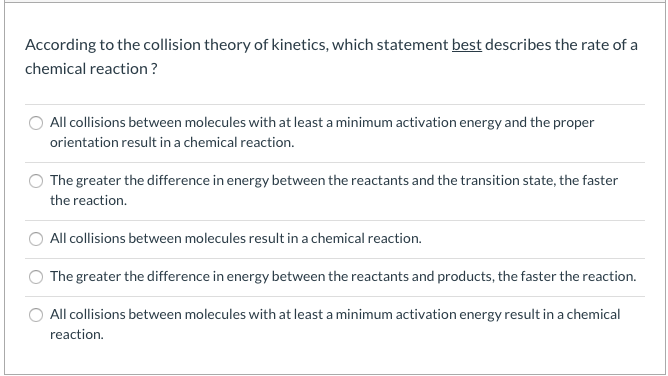



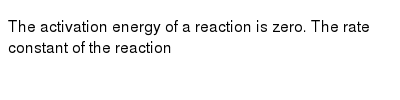














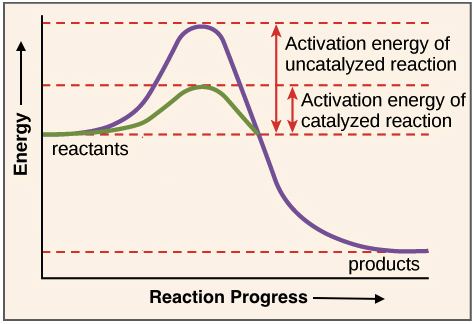

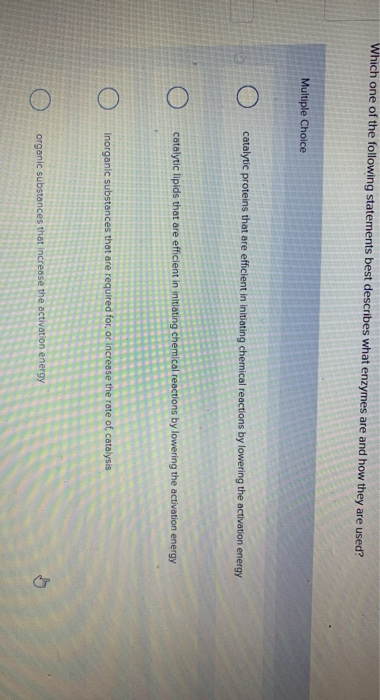
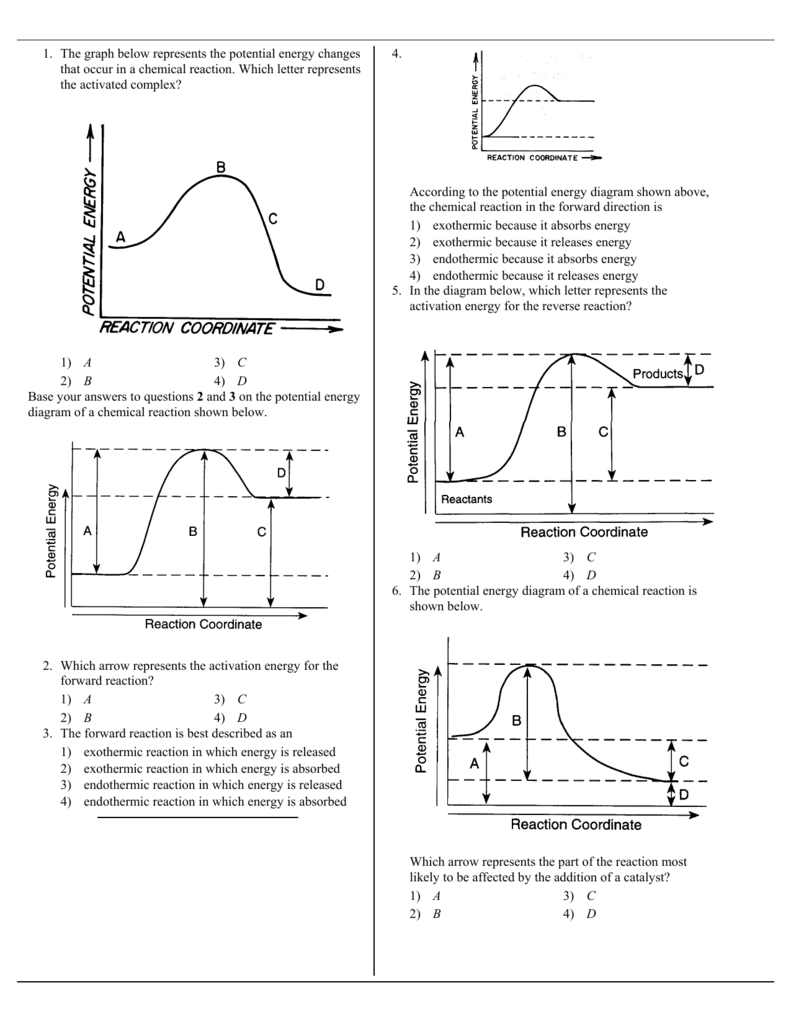



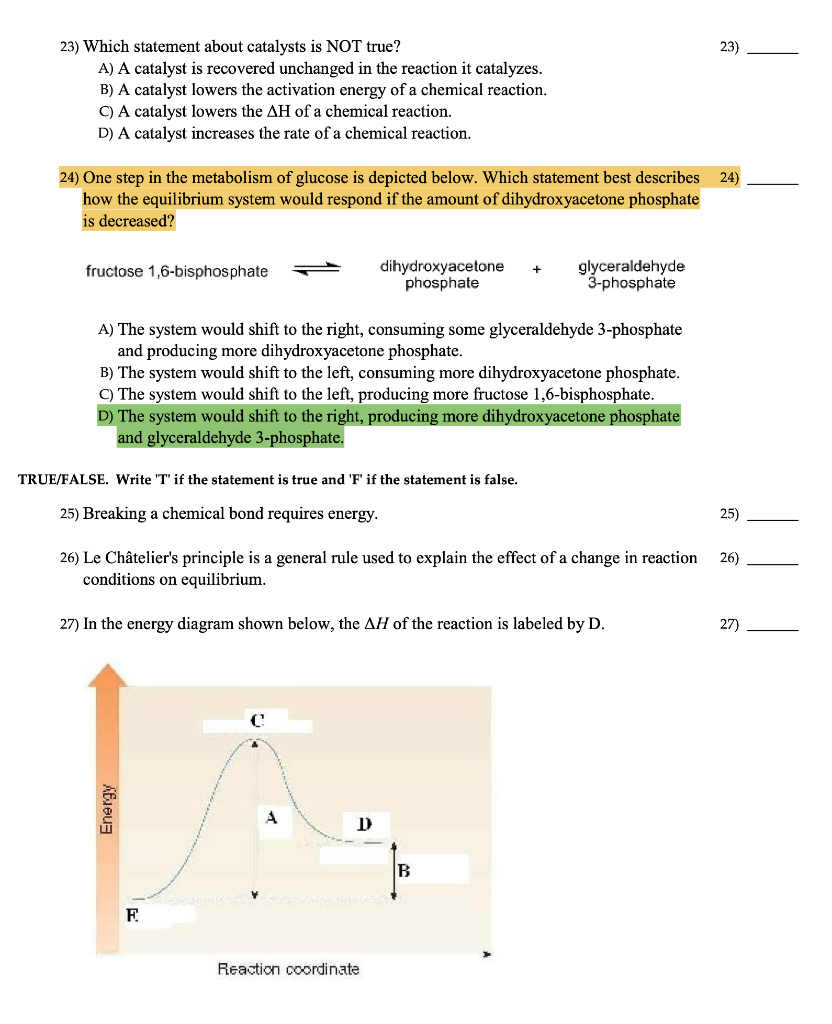



Posting Komentar untuk "Which Statement Best Describes The Relationship Between Activation Energy And Rate Of Reaction"