 Adverse Reactions | Professional Education
Adverse Reactions | Professional EducationWarning: The NCBI website requires JavaScript to operate. Transfusion support for ABO-Incompatible Progenitor Cell TransplantationSummaryABO-incompatible transplants comprise up to 50% of aloginic parent cell transplants. ABO's main, minor and bidirectional transplants have unique complications that may occur, including hemolisi at the time of infusion of parent cells, hemolisis during donor grafting, passenger lymphocyte syndrome, delayed red blood cell graft graft and pure red cell aplasia. Proper support for transfusion during the different phases of the alogenic stem cell transplant process is an important part of ABO's incompatible transplant. Introduction ABO antigens are oligosaccharides that are formed by adding sugars to a precursor substance located on the surface of the cells. They are present in a series of cells other than red blood cells, including vascular endothelial cells. ABO antigens may also be present as soluble substances in body fluids. Individuals who do not express antigens A or B will make antibodies or isoagglutinins to the antigen (s) that do not express []. On the contrary, Rh antigens are only present in red blood cells. Rh antibodies only form after exposure to Rh antigen through transfusion or pregnancy []. Because HLA genes are located in the short arm of chromosome 6, ABO genes are found in the long arm of chromosome 9 and Rh genes are found in the short arm of chromosome 1, even HLA-matched brothers will often be ABO-incompatible. The only guarantee to find two brothers who are ABO-, Rh- and HLA-matched is in twins. If there is a donor of stem cells approved by the ABO, the donor is considered to be preferable to a donor not matched by the ABO. However, other considerations, including the type of HLA, the age of donors and the CMV state, are considered more important in the selection of parent cell donors than the ABO party. In about 40-50% of the stem cell transplants, the donor and the receiver are incompatible ABO. ABO's incompatible parent cell transplants can be classified as main incompatible ABOs, less incompatible ABO or bidirectional ABOs []. Transfusion during the transplantation of parent cells can be separated in three phases, with phase I that occurs during the preparation regime, phase II during grafting and phase III after grafting. How to safely transfuse patients who receive ABO's incompatible parent cell grafts is an important part of the transplant process. Major ABO Incompatibility A parent cell graft is classified as the main incompatible ABO if the receptor has preformed antibodies or isoagglutinins directed against the red blood cells of the graft. This occurs in all patients in the blood group or who received a graft or not a group. It also occurs in blood group A and B patients who receive an AB group graft (table). Significant transfusion-related complications may occur in the receptors of the main injertos of incompatible parent cells of ABO. The first complication that can occur is hemolisi at the time of infusion, because the graft contains incompatible red blood cells and the receptor has preformed antibodies directed against these cells []. The reaction is identical to a hemolytic transfusion reaction that can occur from the transfusion of an incompatible unit of red blood cells. Studies have shown that the risk of hemolysis increases with an increase in the volume of transfused incompatible cells. However, there is no volume of incompatible red blood cells that can be considered completely safe to infuse []. The amount of red blood cells present in a graft of parent cells varies according to the type of collection. Aféesis products usually contain less than 20 ml of red blood cells. Afheresis products can be infused in adult patients without the need to reduce the amount of red blood cells in the product. However, it may be necessary to reduce red blood cells in the product for pediatric patients and young adults. Bone marrow products usually have a 25-35% hematocrit, which means that 1 l bone marrow can contain up to 350 ml of red blood cells, which is more than a packaged red blood cell unit. Therefore, bone marrow products that are incompatible ABO will usually have to be manipulated to reduce the number of red blood cells before infusion. Cord blood products have a similar hematocrit, but the removal of red blood cells before the storage of products limits the number of incompatible red blood cells that are present in most components. The volume of red blood cells present in any parent cell product may significantly decrease by using a cell-processing device and an agent that helps cell sedimentation []. However, the reduction of red blood cells will also result in the loss of parent cells. An alternative to the reduction of the number of red blood cells present in the graft is the reduction of isoagglutinins in the receptor. However, this is not often done in the United States. Pure inference of therapeutic aféesis to prevent hemolysis in an incompatible transplant important ABO has been classified as an indication of category II (disorders for which aféesis is accepted as second-line therapy, either as independent treatment or in conjunction with other treatment modes) for the exchange of plasma by the American Society for Aferesis [].Table 1Type of cells PRCA is defined as reticulocytopenia (The concerns about transfusion in the main incompatible parent cell transplants ABO are the compatibility of transfused red blood cells and restricting the transfusion of additional isoagglutinains incompatible by donors. During phase I of the transplant process, the receptor may safely receive blood components of the receptor type. During phase II, the compatibility of red blood cells can be addressed through the transfusion of receptor-type cells. This is safe because receptor red blood cells will always be supported in a major ABO-compatible stem cell transplant. In order to reduce the additional transfusion of isoagglutinins directed against the grafted red blood cells, the platelets and the donor-type plasma are typically transfused in phase II. For example, if the receiver is type of blood O and the donor is type of blood A, the transfusion would be with type O red blood cells and type A platelets and plasma. In practice, it may not always be possible to provide the preferred type of platelets due to supply constraints. For this reason, transfusion services supporting the transplant of parent cells should define what type of platelets should be administered if the first option of platelets is not available. Table lists that platelets should be administered, in order of preference, if there is no donor type platelets. Additional information on the transfusion of platelets in the transfusion of platelets in the transplant of parent cells is provided in the section. BABORITY, BABROBODY, BABORITIES During phase III of the transplant, donor red blood cells have grafted, and incompatible receptor isoagglutinins are no longer present. The receiver can receive red blood cells, platelets and donor-type plasma. Less ABO Incompatibility A parent cell transplant is classified as a less incompatible ABO when the donor's plasma contains isoagglutinins directed against ABO antigens present in the receptor's red blood cells. This occurs when a receptor that is a blood group A, B or AB receives a parent cell graft from a group O donor or when an AB group receptor receives a graft from a donor that is a blood group A or B (Table). Significant complications that can be associated with a less than ABO-compatible stem cell transplant include hemolysis of the receptor's red blood cells at the time of infusion and delayed hemolysis of the receptor's red blood cells secondary to the stimulation of donor's B cells. The immediate hemolysis secondary to passive infusion of isoagglutinins donors, directed against the receptor red blood cells, is not common. The risk is increased with components containing large volumes of plasma, in donors with high-distance isoagglutinains, and in receptors with relatively small blood volumes []. This risk can be reduced by reducing the plasma volume in the parent cell product. In general, this is likely to be necessary in bone marrow products, which may contain significant amounts of donor plasma. The delayed hemolysis after a minor ABO transplant can be fatal and usually starts within 7-10 days after the transplant. Hemolysis is secondary to antibodies, directed against native red blood cells of the receptor, formed by B lymphocytes that are contained within the graft of parent cells, and is called passenger lymphocytes syndrome. This complication has not been reported after the cord blood transplant, which is most likely explained by the fact that B lymphocytes within the cord blood would not have previously been sensitized to ABO antigens. Isoagglutinines to ABO antigens do not begin to develop until several months after birth []. Transfusion needs should focus on transfusion of donor-received red blood cells while avoiding transfusion of isoagglutinins directed against residual red blood cells of receptor origin. It seems logical that reducing the amount of incompatible red blood cells present in the receptor, before the transplant, would reduce the risk of passenger lymphocyte syndrome. Therefore, some have suggested that the prophylactic exchange of red blood cells before the minor incompatible stem cell transplant should be routinely performed []. However, a recent study of the exchange of red blood cells before the incompatible ABO transplant in the author ' s own institution suggested that this practice does not diminish severe hemolisis. In addition, it did not improve the survival of 1 year, the number of transfused red blood cells after the transplant, or the duration of the hospitalization []. The American Society of Aferesis assigns the prophylactic exchange of red blood cells before the minor transplant of incompatible stem cells of ABO a classification of category III (The optimal role of aféesis therapy is not established. Decision-making should be individualized) []. In phase I of the transplant process, all the blood components received by the receptor can be of a receptor type. However, in some centers, it is practical to transfuse donor-type red blood cells as soon as it is known that the patient is likely to receive a lesser ABO incompatible parent cell transplant. The reasoning behind this is similar to the reasoning behind the prophylactic exchange of red blood cells and is to reduce the amount of red blood cells type receptors that can be hemolyzed if the syndrome of passenger lymphocytes develops. Although this may seem logical, there are no controlled clinical trials to support this practice. In addition, the volume of hemolysis experienced in cases of passenger lymphocyte syndrome may significantly exceed the volume of the receptor's red blood cells []. It has been hypothesized that the ABO antigens of the destroyed red blood cells adhere to the donor-type transfused red blood cells that can cause transfused red blood cell hemolysis in addition to the hemolysis of the receptor-type cells. In phase II of the transplant process, the patient must receive donor-type red blood cells. Platelets and plasma should not ideally contain isoagglutinains directed against the receptor's red blood cells. Platelets and plasma of the receiver's type of blood are considered the first option. For example, if the receptor is blood group A and the donor is blood group O, the transfusion should be with group O red blood cells and with group A platelets and fresh frozen plasma. In phase III of the process, many centers transfer red blood cells, platelets and donor-type plasma. An alternative approach to transfusion of platelets and plasma that considers isoagglutinin compatibility with the donor and receiver is preferred by some transplant centers []. ABO antigens are expressed in many other cells, including endothelial cells. Cells, in addition to red blood cells, continue to express receptor-type ABO antigens after the transplant of parent cells []. The selection of platelets and plasma products compatible with the donor and receptor in phase III of the transplant will minimize the transfusion of isoagglutinains directed against the ABO antigens of the native endothelial cells of the receptor. The centers that favor this approach would transfuse the donor-type red blood cells, but continue to transfuse platelets and plasma of the receptor type (first choice) in phase III (Table) as isoagglutinans in donor-type platelets and plasma would be incompatible with the ABO antigens expressed in the endothelial cell of the receptor. Bidirectional ABO IncompatibilityBidirectional Incompatibility ABO incompatibility occurs when both ABO's main incompatibility and ABO's minor incompatibility are present in the same transplant. This occurs when a group A receptor receives a parent cell transplant from a group B donor and when a group B receptor receives a graft from a group A donor (table ). All of the complications described above associated with the transplantation of primary and incompatible parent cells of ABO are associated with ABO's incompatible bidirectional transplantation, including donor cell hemolisis at the time of infusion, receptor cell hemolysis at the time of infusion, grafting, PRCA, and passenger lymphocyte syndrome. Progenitor cell products, in particular bone marrow, may need to have red blood cells and plasma removed before administration. In phase I of the transplant, the patient may receive all products of the type of blood of the receptor. During phase II of the transplant, only red cells of group O can be transferred because all other types of red blood cells would be incompatible with the donor, the receiver or both. The first option of platelets and plasma is the group AB. All other types of platelets and plasma will contain isoagglutinins that will be incompatible. If the first option of the platelets is not available, the platelet preference order is listed in the table. In phase III of the transplant, transfusion of all products can be of a donor type. Alternatively, if you prefer isoagglutinin compatibility with both the donor and the receiver, the transfusion of red blood cells can be of a donor type while the first choice of platelets and remains of plasma group AB. Additional ABO-Incompatible Transplant Executing an additional transplant after the failure of the first graft or after repetition of diseases has become more common. In the case of failure of the first graft of parent cells, there is often no evidence of the first graft, and only two types of blood, the type of native blood of the patient and the type of blood of the second parent cell donor should be considered. In cases where the patient is transplanted for the recurrence of the disease, there may be three types of blood involved; the original type of blood of the patient, the type of blood of the first donor and the type of blood of the second donor. In these cases, the simplest way of transfuming the patient after the infusion of the second graft is to provide red blood cells of group O and group AB and plasma platelets until the graft of the second donor is established. If there are no AB group platelets, the transfusion of platelets is preferred to protect the graft of the second donor. Transfusion of platelets In an ideal world, the first option of transfusion of platelets would be available to all patients, including patients with stem cell transplantation. In fact, because platelets in the US only have a 5-day life, the first choice of platelets is not always available, and the patient cannot wait for transfusion until the first option is available. Table lists, in order of preference, the blood group of platelets that should be used if the first option is not available. Preferences are determined on the basis of minimizing the transfusion of incompatible isoagglutinins to protect the graft. Therefore, if incompatible platelets should be transfused, it is preferable to administer platelets with incompatible isoagglutinins directed against the receiver's ABO antigens. Other ways of minimizing the transfusion of incompatible isoagglutinains should also be considered. Some donor centres and transfusion services limit exposure to incompatible isoagglutinains by limiting donation of aféesis platelets by donors with high-level isoagglutinains or limiting transfusion of collected units from these donors to compatible receptors []. For example, in the institution of the author, aféesis platelets that have a positive titer in a dilution 1:100 for anti-A or anti-B can only be transfused to compatible receptors. Another means of limiting exposure to incompatible isoagglutinins is the use of platelets stored in a platelet-additional solution (PAS). The current PAS platelets approved for use in the US have a 65% less plasma than conventional aféesis platelets, and presumably a 65% less isoagglutinains. In addition, PAS models have been shown to reduce the incidence of allergic transfusion reactions by 45% []. Although allergic transfusion reactions are often mild, they can cause significant discomfort of the patient and are avoided better, if possible. Processes and PoliciesParent cell transplant organizations should have policies and procedures to address many aspects of transfusional medicine. Transfusion service should be informed that the transplant will take place. Ideally this should happen as soon as possible in the process. Transfusion service should have a method to determine which blood components the patient should receive at each stage of the transplant. In addition to the ABO, policy should address transfusion practice if the donor and the receiver are incompatible. Many installations opt to transfuse Rh-negative red blood cells to phase III of the transplant, changing to positive Rh red blood cells at this time, if the donor is Rh-positive. Transfusion practices can be formalized in a standard operating procedure (SOP) that is followed for each transplant. Alternatively, a transfusion service doctor may perform a consultation that is placed in the patient's medical registry and used in the transfusion service. This option has the benefit of having a transfusion medicine doctor check each case of bone marrow transplant before transplantation and individualize transfusion therapy for each patient. If the consultation is placed in the patient's medical register, it will also be available for members of the clinical team. Another policy to be established is how to determine when phase III of the transplant begins. There are no absolute rules governing this decision, and practice is varied []. Thus, most transfusion services will have to establish their own policies in the area. At the very least, the patient's advanced blood type (antigens expressed in the red blood cells) must be donor type, and the receptor isoagglutinins directed against the donor's red blood cells must have disappeared. Other factors that can be considered when making the determination to change donor-type red blood cells for transfusion include transfusion requirements and the results of chimerism studies. The individual review of the transfusion requirements of each patient's red blood cells and the stability of donor chimerism can help make the decision to change to the transfusion of donor-type red blood cells. In the author ' s institution, there is a minimum requirement that the patient ' s type of progress is donor and there are no incompatible isoagglutinains present in two consecutive samples taken at least one week apart to consider changing the patient ' s type of blood. The case is reviewed by a transfusion medicine doctor who considers the patient's clinical state, transfusion requirements and the results of chimerism before deciding whether it is appropriate to change the patient's blood type. In general, we tend to be more conservative about changing the type of blood of the patient in cases of ABO incompatibility greater than in cases of ABO incompatibility. Additionally, we need a written order and signed by a transfusion medicine doctor to change the patient's blood type in our blood bank computer system. A policy on the transplantation of parent cells and the irradiation of blood components should be established to prevent grafting associated with transfusion against the disease (TAGVHD). The irradiation of cell blood components should begin at the time the conditioning regime begins. Plasma and cryoprecipitate do not need to be irradiated because they are acelaus. Transfusion services should determine how long after transplantation the patient will continue to receive irradiated cell blood components. AABB (formerly American Blood Bank Association) recommends that a transfusion service have a policy regarding the transfusion of irradiated components and that cell blood components be irradiated when a patient is identified as a risk of TAGVHD []. In practice, many centers continue to provide irradiated products for this group of patients for the rest of their lives. Dissemination statement The author has no conflicts of interest to report. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Accessibility links Search results Results webCH 17 blood Flashcards The ← QuizletChapter 17 - Blood allocation Flashcards A case of incompatible blood transfusion ABO treated by ... Immunological risks of whole blood: ABO compatibility, D ...Transfusion Cell support Progenitors ABO-Incompatible ...Blood and Blood Transfusion Products: Indications and ...Anatomy & Physiology Chapter 17 - Vocabulary Blood WARNING Easy ... a misadjustment of blood types during dangerous ais because _________ Which of these develops from lymphoid stem cells? Which of the deformed elements is the most ungrateful concentration? How many oxygen molecules can be transported by a hemoglobin molecule?A patient has a suspicious-compatible electrolyte imbalance in a blood centrifugal sample, what should not be an inplasm of sampling? Navigation page1 Foot links

Pre-transfusion Testing | Professional Education
Adverse Reactions | Professional Education
Adverse Reactions | Professional Education
Adverse Reactions | Professional Education
Understanding the Impact of Platelet ABO Matching – Part 1: The Basics | ThromboLUX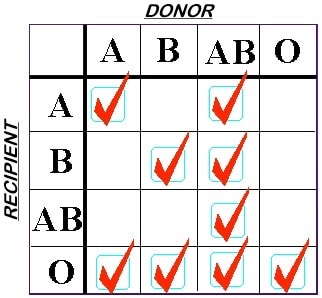
Breaking the Rules; Group A Plasma in Emergencies - Blood Bank Guy
Solved: -21) An Incompatible Blood Transfusion, Such As Ty... | Chegg.com
ABO incompatibility does not influence transfusion requirements in patients undergoing single-unit umbilical cord blood transplantation | Bone Marrow Transplantation
Solved: QUESTION 6 Which Of The Following Is Normally Foun... | Chegg.com
CDHB Red Book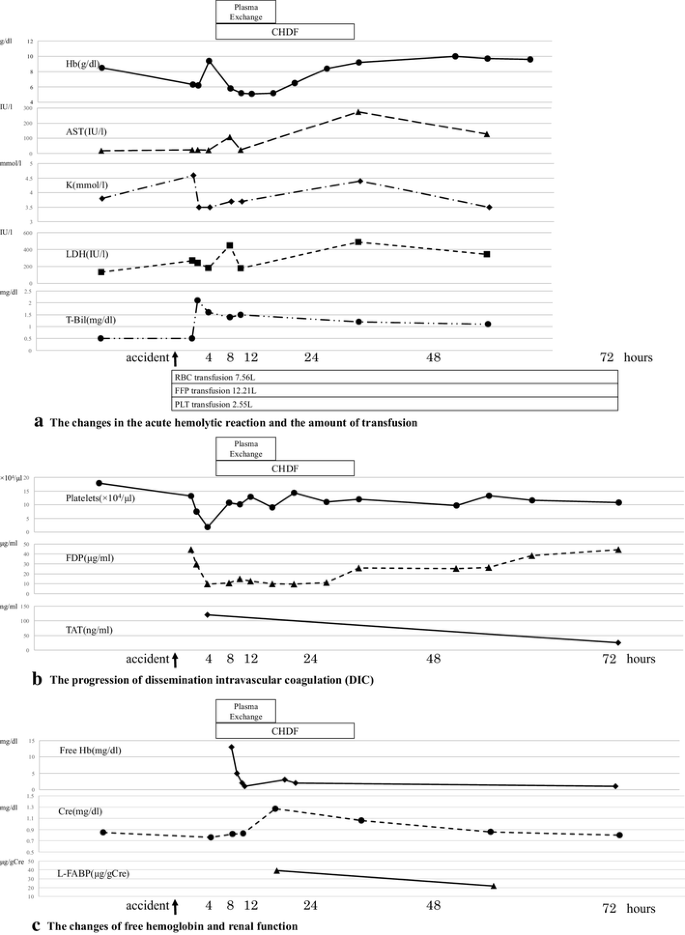
A case of ABO-incompatible blood transfusion treated by plasma exchange therapy and continuous hemodiafiltration | SpringerLink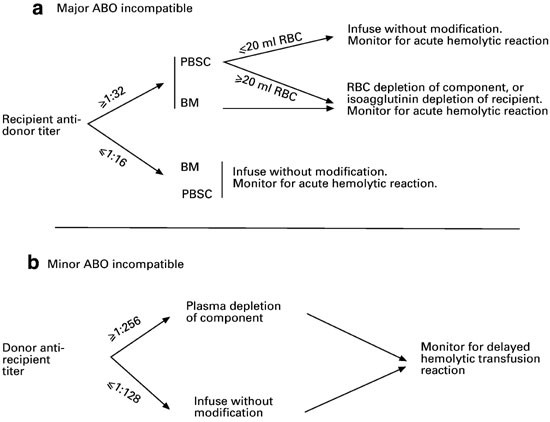
Hematopoietic stem cell transplantation between red cell incompatible donor–recipient pairs | Bone Marrow Transplantation
Immunologic risks of whole blood: ABO compatibility, D alloimmunization, and transfusion‐related acute lung injury - Harm - 2019 - Transfusion - Wiley Online Library
Adverse Reactions | Professional Education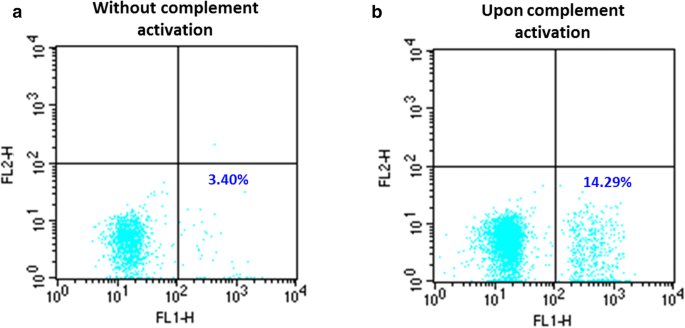
Complement activating ABO anti-A IgM/IgG act synergistically to cause erythrophagocytosis: implications among minor ABO incompatible transfusions | Journal of Translational Medicine | Full Text
Hematopoietic stem cell transplantation between red cell incompatible donor–recipient pairs | Bone Marrow Transplantation
PDF) The least incompatible crossmatch red blood cell transfusion by biological compatibility test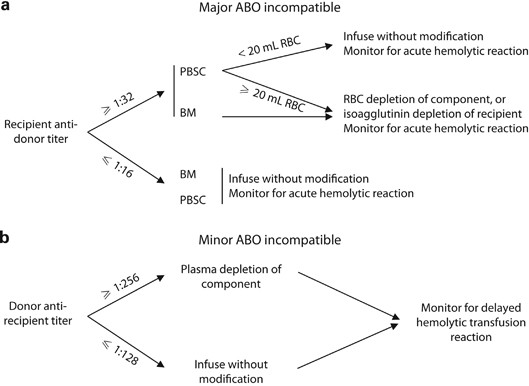
Red blood cell-incompatible allogeneic hematopoietic progenitor cell transplantation | Bone Marrow Transplantation
A Guide to Transfusion Medicine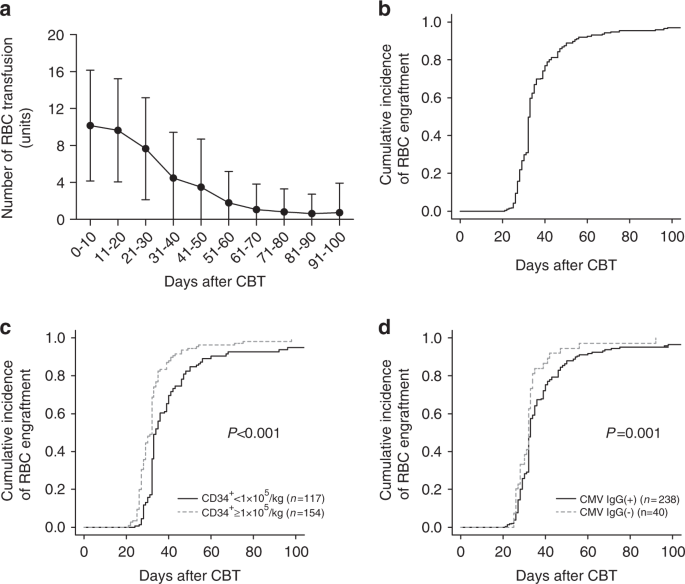
Red blood cell transfusion burden by day 30 predicts mortality in adults after single-unit cord blood transplantation | Bone Marrow Transplantation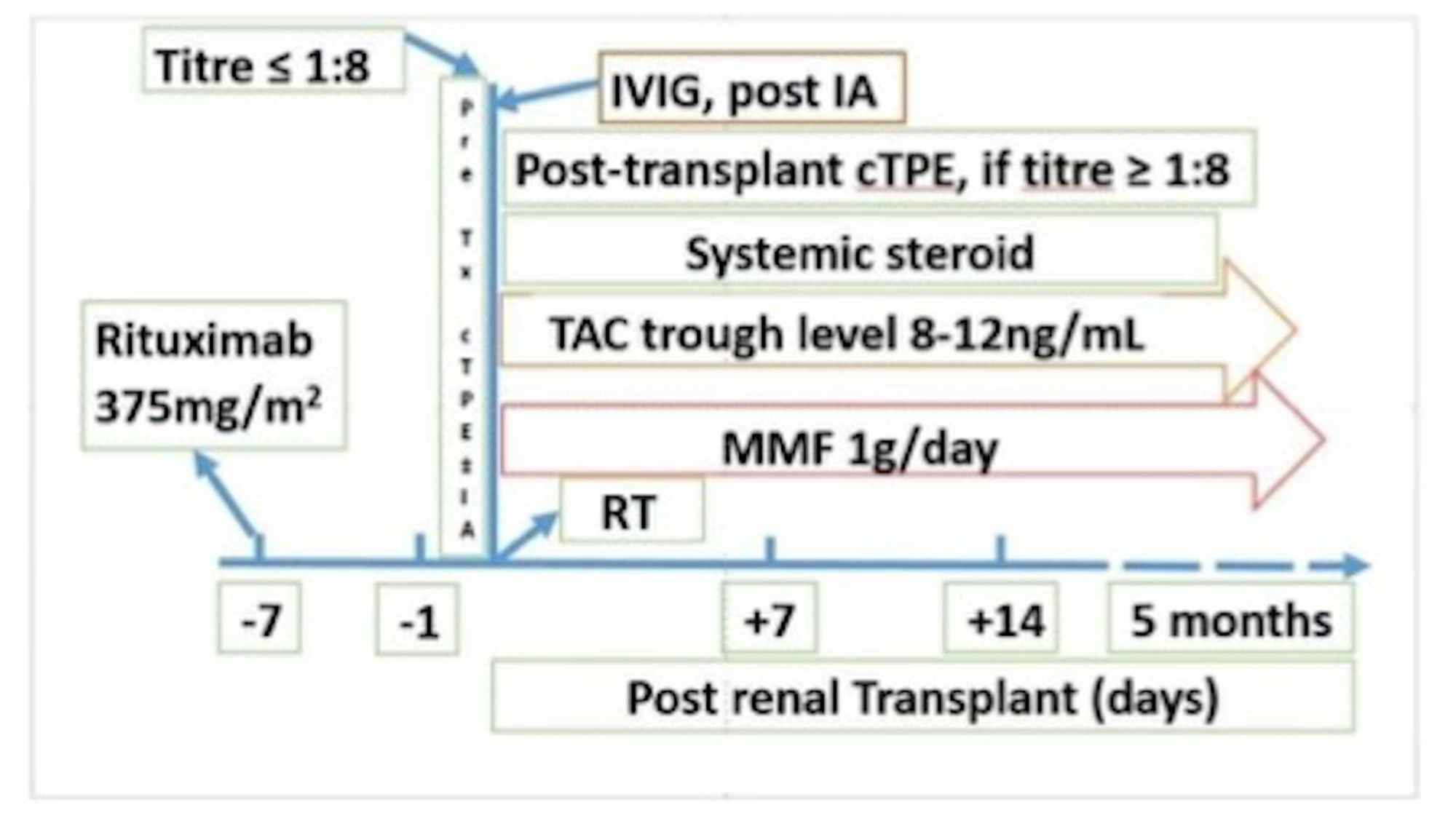
Cureus | Therapeutic Immunoadsorption and Conventional Plasma Exchange in ABO-incompatible Renal Transplant: An Exculpatory Evidence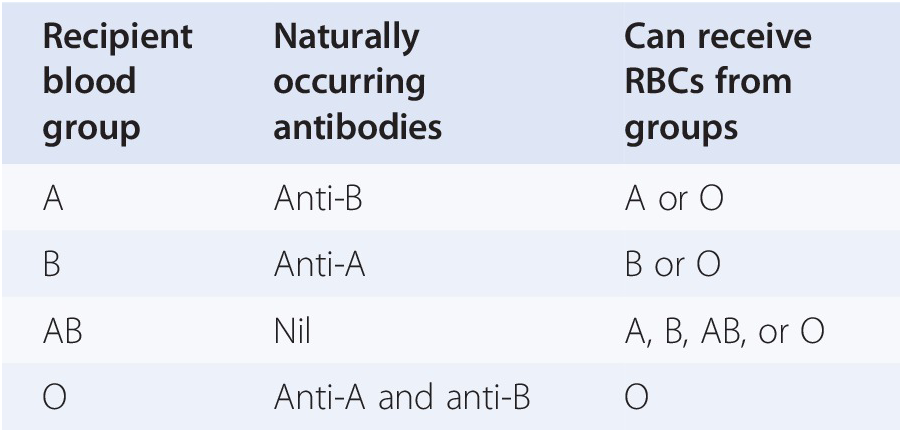
Blood Transfusion: Indications and Safe Use in Children (Chapter 34) - Pediatric Hematology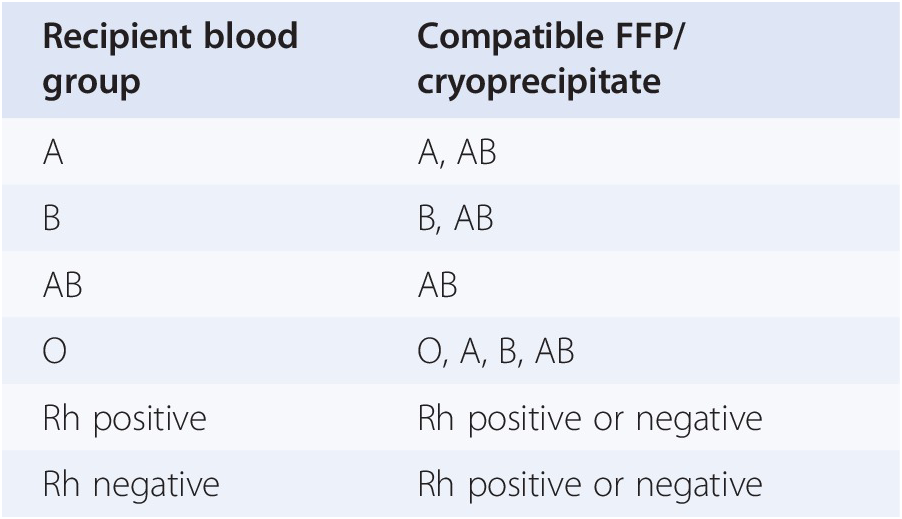
Blood Transfusion: Indications and Safe Use in Children (Chapter 34) - Pediatric Hematology
Role of ABO and Rh Type in Platelet Transfusion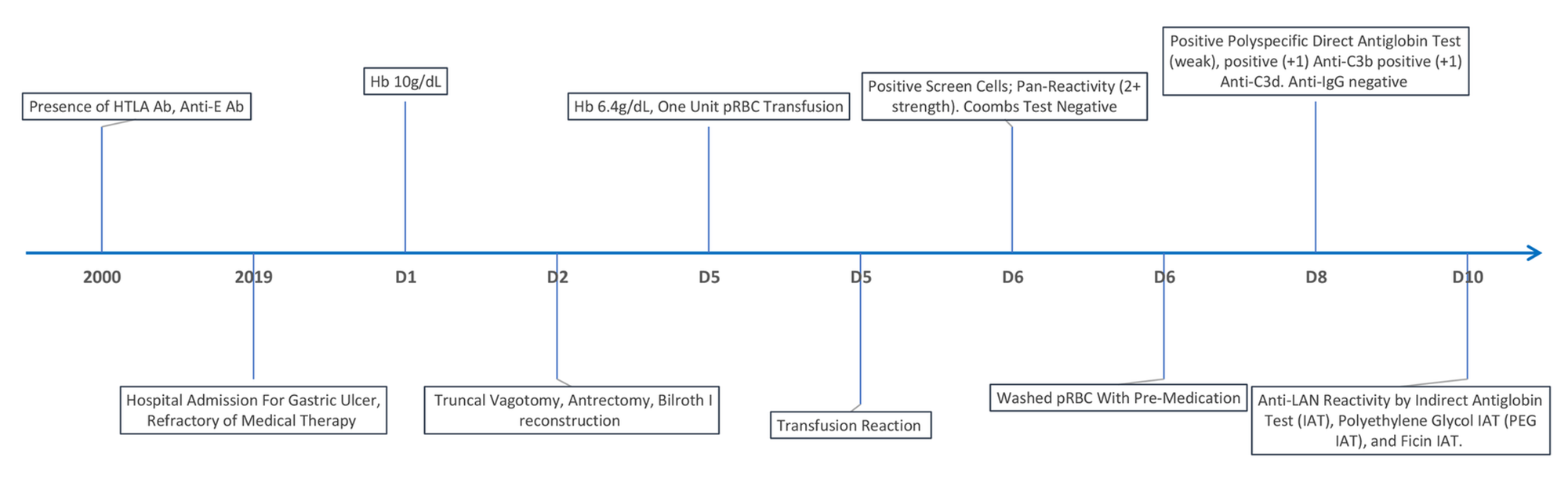
Cureus | Anti-Lan Antibodies: A Rare Etiology of Severe Blood Transfusion Reaction![Solved: 66. The]()
Solved: 66. The "memory Component Of Our Immune System Acc... | Chegg.com
Can doctors transfuse a wounded patient who had lost a lot of blood with incompatible blood type, if the right blood type is not available, in order to save the patients life? -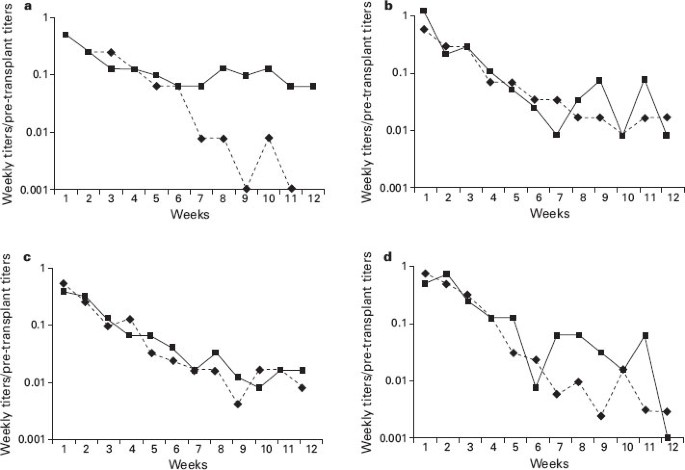
Transplantation of ABO-incompatible bone marrow and peripheral blood stem cell components | Bone Marrow Transplantation
Recurring Errors in Transfusion and Human Factors
A Guide to Transfusion Medicine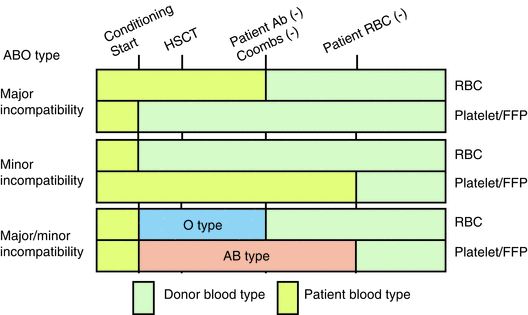
Transfusion | SpringerLink
Platelet Transfusion, Alloimmunization and Management of Platelet Refractoriness | Professional Education
PDF) Complement activating ABO anti-A IgM/IgG act synergistically to cause erythrophagocytosis: implications among minor ABO incompatible transfusions
Solved: QUESTION 19 1 Points Save Answer In A Transfusion ... | Chegg.com
Safety considerations and risks of transfusion - Anaesthesia & Intensive Care Medicine![Full text] Autoimmune hemolytic anemia: transfusion challenges and solutions | IJCTM Full text] Autoimmune hemolytic anemia: transfusion challenges and solutions | IJCTM](https://www.dovepress.com/cr_data/article_fulltext/s81000/81870/img/IJCTM_81870_F001.jpg)
Full text] Autoimmune hemolytic anemia: transfusion challenges and solutions | IJCTM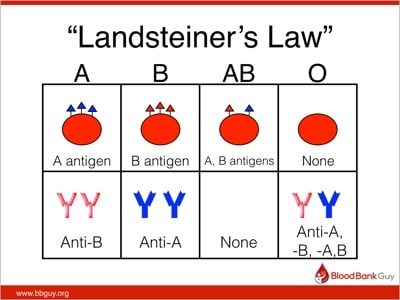
Breaking the Rules; Group A Plasma in Emergencies - Blood Bank Guy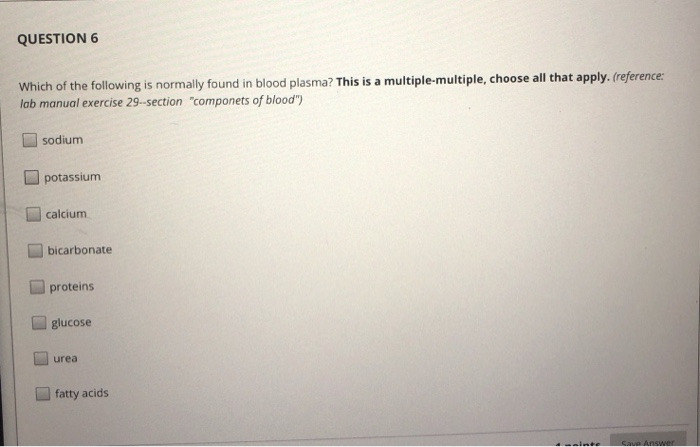
Solved: QUESTION 6 Which Of The Following Is Normally Foun... | Chegg.com
ABO-Incompatible Kidney Transplant Outcomes | American Society of Nephrology
Transfusion of Blood and Blood Products: Indications and Complications - American Family Physician
 Adverse Reactions | Professional Education
Adverse Reactions | Professional Education




























![Full text] Autoimmune hemolytic anemia: transfusion challenges and solutions | IJCTM Full text] Autoimmune hemolytic anemia: transfusion challenges and solutions | IJCTM](https://www.dovepress.com/cr_data/article_fulltext/s81000/81870/img/IJCTM_81870_F001.jpg)




Posting Komentar untuk "choose the incompatible transfusion."